Chlorine definitely evaporates overnight, and can easily be driven out by boiling. Chloramine, however, is somewhat more persistent: it doesn't come out by itself. That half campden tablet eliminates chloramine instantly.Any kind of water filter meant to eliminate chlorine will reduce 95%+ Instantly. The remaining <5% is probably negligible. Also, chlorine evaporates fairly rapidly when left in an open container. My practice is to fill my AIO the night before and leave it open. But, for cheap insurance I do add a 1/2 campden tablet.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Star San
- Thread starter JoshuaGates
- Start date
-
- Tags
- before bottling star san
- Joined
- Mar 14, 2018
- Messages
- 10,918
- Reaction score
- 20,459
- Points
- 113
Chloromine however, as mentioned, is much more stable, your municipal water supply company will be able to tell you which they use.Any kind of water filter meant to eliminate chlorine will reduce 95%+ Instantly. The remaining <5% is probably negligible. Also, chlorine evaporates fairly rapidly when left in an open container. My practice is to fill my AIO the night before and leave it open. But, for cheap insurance I do add a 1/2 campden tablet.
- Joined
- Aug 12, 2020
- Messages
- 60
- Reaction score
- 20
- Points
- 8
What rate of star san do you use per liter of water , the star san bottle i have has no rates on it
- Joined
- Mar 31, 2021
- Messages
- 4,266
- Reaction score
- 6,345
- Points
- 113
I think it is 2oz per 5gallons, However that converts to litersWhat rate of star san do you use per liter of water , the star san bottle i have has no rates on it
- Joined
- Jun 27, 2019
- Messages
- 2,776
- Reaction score
- 8,485
- Points
- 113
1 oz per 5 gallons.I think it is 2oz per 5gallons, However that converts to liters
https://fivestarchemicals.com/star-san
My bottle says 1 ounce per 5 gallons.What rate of star san do you use per liter of water , the star san bottle i have has no rates on it
- Joined
- Nov 23, 2023
- Messages
- 36
- Reaction score
- 93
- Points
- 18
This is what I did for my most recent batch. I used my usual filtered water left out overnight, followed by campden. Kegging it today. I'm not expecting any issues.Any kind of water filter meant to eliminate chlorine will reduce 95%+ Instantly. The remaining <5% is probably negligible. Also, chlorine evaporates fairly rapidly when left in an open container. My practice is to fill my AIO the night before and leave it open. But, for cheap insuranIce I do add a 1/2 campden tablet.
- Joined
- Apr 29, 2021
- Messages
- 1
- Reaction score
- 2
- Points
- 3
If this continues then I would suggest tasting the beer before bottling, if the taste is already present then it's something that happens before bottling. I usually guzzle down my hydrometer sample at that point just to get an idea of how it's going 
off flavor from lme or dme perhaps?
- Joined
- Apr 30, 2024
- Messages
- 7
- Reaction score
- 6
- Points
- 3
Coming back to this. I'm still brewing y'all! 
So, I'm 100% convinced it's my water. If I start from RO/DI water, I have to add the "nutrients/minerals" to create the water chemistry the brew requires, correct?
Now, the new question. How the heck do I do that? Can someone point me in the right direction, or provide an article, that outlines the water chemistry process?
Thanks everyone!
So, I'm 100% convinced it's my water. If I start from RO/DI water, I have to add the "nutrients/minerals" to create the water chemistry the brew requires, correct?
Now, the new question. How the heck do I do that? Can someone point me in the right direction, or provide an article, that outlines the water chemistry process?
Thanks everyone!
It could be chlorine, but it could also be the "box kit flavor" iv tried a lot of homebrew and there is a noticable flavor difference between all grain and extracts.
I personally wouldnt bother with RO, I brew without any filtration on beach water without any issues. I get my water tested regularly and it is pretty decent. I throw 20g CaCl2 and 20g Gypsum into every recipe and let it ride.
Getting your water tested is not super expensive and can help a lot with water chemistry.
I personally wouldnt bother with RO, I brew without any filtration on beach water without any issues. I get my water tested regularly and it is pretty decent. I throw 20g CaCl2 and 20g Gypsum into every recipe and let it ride.
Getting your water tested is not super expensive and can help a lot with water chemistry.
campden tablets are going to be the easiest route.Coming back to this. I'm still brewing y'all!
So, I'm 100% convinced it's my water. If I start from RO/DI water, I have to add the "nutrients/minerals" to create the water chemistry the brew requires, correct?
Now, the new question. How the heck do I do that? Can someone point me in the right direction, or provide an article, that outlines the water chemistry process?
Thanks everyone!
you can also get your water tested.
If you dont trust your water you could buy spring water and just roll with whatever it is and not need to mess with salts.
just my 2 cents
- Joined
- Mar 31, 2021
- Messages
- 4,266
- Reaction score
- 6,345
- Points
- 113
That is like asking "what car should i buy" lolComing back to this. I'm still brewing y'all!
So, I'm 100% convinced it's my water. If I start from RO/DI water, I have to add the "nutrients/minerals" to create the water chemistry the brew requires, correct?
Now, the new question. How the heck do I do that? Can someone point me in the right direction, or provide an article, that outlines the water chemistry process?
Thanks everyone!
There are hundreds of books on brewing water. But if you follow the water profile, starting from RO. It should point you in the right direction.
You can get all the different brewing salts on amazon pretty cheap.
- Joined
- Jul 4, 2022
- Messages
- 3,071
- Reaction score
- 5,053
- Points
- 113
Publix spring water is what I use, and I like my beer.campden tablets are going to be the easiest route.
you can also get your water tested.
If you dont trust your water you could buy spring water and just roll with whatever it is and not need to mess with salts.
just my 2 cents
- Joined
- Mar 14, 2018
- Messages
- 10,918
- Reaction score
- 20,459
- Points
- 113
I personally use calcium chloride, gypsum, Epsom, and pickling/Canning salt. For lighter beers a bit of lactic acid will most likely be needed to bring pH down. I was always forgetting to add the acid to the mash so I now use a small amount of acidulated malt in the grist instead. For a dark beer I find it necessary to use a bit of baking soda to bring pH up. When designing your beer start with making sure the the calcium is up near 100ppm. The other two keys are sulfates and chlorides, you will want to balance these depending on what you are brewing. A "balanced profile" would have similar levels of each, which will work well with blonde ales, and others where you don't want to accentuate the malts, or the hops specifically. Higher sulfates will will accentuate hops, and higher chlorides will accentuate malts. Oddly for a Hazy IPA (NEIPA) you will want to use a profile with higher chlorides, like a 3:1 ratio, like as much as say 225:75Coming back to this. I'm still brewing y'all!
So, I'm 100% convinced it's my water. If I start from RO/DI water, I have to add the "nutrients/minerals" to create the water chemistry the brew requires, correct?
Now, the new question. How the heck do I do that? Can someone point me in the right direction, or provide an article, that outlines the water chemistry process?
Thanks everyone!
Some magnesium is good for yeast health, but keep that fairly low like 5-10, sodium can be a bit higher than that, maybe 10-20.
You will find it a bit of a balancing act as each additive brings more than one ion to the party.
It doesn't have to be complicated, and it doesn't have to be exact.
- Joined
- Mar 12, 2017
- Messages
- 2,699
- Reaction score
- 5,610
- Points
- 113
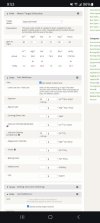
You can use the Water Chemistry Calculator available on this site. You can access it from the Tools pulldown menu or from the water chemistry section when editing a recipe. I almost always choose the latter because it brings along your grain bill (which affects pH). In the calculator you enter in your source water and your target profile. Either of the Balanced profiles are good to use for most beers. Now comes the trial & error part. You enter in various amounts of this or that salt and observe how close it gets you to your target. Some salts alter more than one ion. Adding one salt might put one ion where you want it but another may be out of whack. In the example below you can see adding 8 grams of Gypsum put my calcium close to target but my Sulfate (SO4) is too high. Also my sodium is a little low as well. So, I could back off on the Gypsum addition and add Calcium Chloride to get everything close. And just focus on getting reasonably close. Don't stress about hitting the targets exactly. You'll drive yourself insane.

On a recent podcast (Experimental Brewing I think) they mentioned Spring Water can be problematic in that, if it really is from a spring, it will have an unknown amounts of minerals in it already. But, I've not heard of anyone complaining about this on the forum so it probably falls into the don't-worry-about-it catagory.
On a recent podcast (Experimental Brewing I think) they mentioned Spring Water can be problematic in that, if it really is from a spring, it will have an unknown amounts of minerals in it already. But, I've not heard of anyone complaining about this on the forum so it probably falls into the don't-worry-about-it catagory.
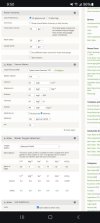
A couple more examples.
My street water pretty much decides that I use balanced profile 1. I add 75% phosphoric acid to adjust ph as needed based on the recipe that I'm doing.
My street water pretty much decides that I use balanced profile 1. I add 75% phosphoric acid to adjust ph as needed based on the recipe that I'm doing.