- Joined
- Mar 14, 2018
- Messages
- 11,295
- Reaction score
- 21,141
- Points
- 113
Kind of a vague subject, but here is some detail as to why I am asking for input.
Any suggestions, or science that can help with this?
Can I use a pressure x surface area of beer x time = x.xx additional volumes?
Should I use a different method of keeping O2 out when cold crashing.
Here is my setup for cold crashing

Here is what the calculator gives me if I am packaging at 3 Celsius.
It is saying that there is already 1.59 volumes in the beer
Thanks in advance for the input!
- I have started using Co2 when cold crashing to prevent O2 ingress, Co2 @ approximately 1-2 PSI
- Planning to bottle the Q2 Dubbel, and want to go on the high end of volumes (2.4)
- Using 500ml Bombers, but also have some 650 Bombers, could use either.
- We know that when cold crashing some Co2 will dissolve back into the beer, and I am adding that little bit of pressure which I assume will increase the amount that dissolves back in.
Any suggestions, or science that can help with this?
Can I use a pressure x surface area of beer x time = x.xx additional volumes?
Should I use a different method of keeping O2 out when cold crashing.
Here is my setup for cold crashing
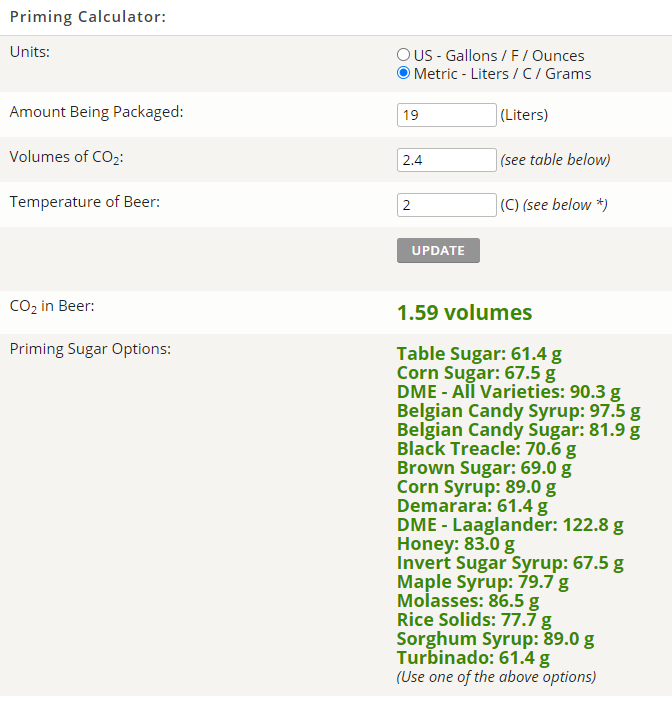
Here is what the calculator gives me if I am packaging at 3 Celsius.
It is saying that there is already 1.59 volumes in the beer
Thanks in advance for the input!









